-
About Us
Keith J. Stine, Chair
Mission Statement
Degree Offerings
Department Photos
Outreach Activities
-
Undergraduate Studies
Undergraduate Degrees
Undergraduate Program Advising
Undergraduate Scholarships and Awards
Research for Undergraduates
Resources
-
Graduate Studies
Graduate Program Overview
Graduate Degrees
Application to Graduate Program
Graduate Awards
Graduate Program Contact Information
-
Faculty
Faculty Contact Information
Inorganic
Organic
Analytical/Physical
Biochemistry
Chemistry Faculty Awards
-
Staff
-
Seminar Programs
Archive
Graduate Student Seminars
Robert W. Murray Lecture
Distinguished Alumni Lecture
-
Departmental News
News Archive
-
Facilities
High Field NMR Facility
MIST Lab
X-Ray Diffraction Facility
-
Alumni Interests
UMSL Chemists
Important Dates
Distinguished Alumni
Alumni Lecturers
-
Contact Information
 |
Dr. Spilling received his B.Sc. and PhD. degrees from the University of Technology, Loughborough, England. He was a Postdoctoral Fellow at Northwestern University and joined the UMSL faculty in 1989. He served as Department Chair from 2004 - 2015 and is now Vice Chancellor for Research and Economic & Community Development. |
cspill@umsl.edu |
Research Interests
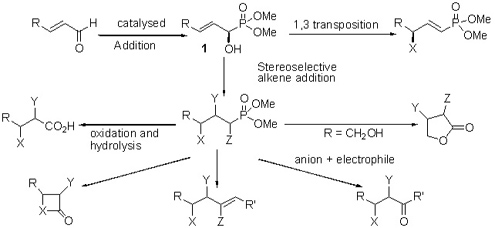
The last decade has seen a rapid expansion in the interest in the asymmetric synthesis of 1-substituted phosphonates. The unique properties of phosphorus provide a fascinating and challenging approach to stereoselective reactions. Our goal is to examine the use of chiral phosphonamides and phosphonates in stereoselective reactions. We reported the first example of a lathanide chiral catalyst in the addition of simple phosphites to achiral aldehydes. More recently, we discovered some promising titanium alkoxide systems. We are attempting to expand the chemistry of allylic hydroxy phosphonamides and phosphonates formed in the chemistry discussed above. Allylic hydroxy phosphonates are similar to regular allylic alcohols and should undergo similar chemistry. However, the presence of the phosphonate significantly alters the electronics of the system, and enables control of both regiochemistry and stereochemistry. Our initial work focused on the palladium catalyzed addition of amines to the carbonate derivatives of allylic hydroxy phosphonates, and several examples of this reaction have been performed. The rearrangement proceeds with complete retention of chirality. A number of 3,3 sigmatropic rearrangements and alkene addition reactions have been studied. The newly developed chemistry of the hydroxy phosphonates is being applied toward the synthesis of the cathepsin B, cruzain, and renin inhibitors, lactones and lactams.
Psammapsylin, fistularin, and the bastadins are related metabolites isolated from sponges found worldwide. This family of highly brominated compounds possess wide ranging biological activity, including anti-HIV activity, and anti-tumor properties. They are related in their biosynthetic origin, as oxidation products of tyrosine. We are exploring biomimetic approaches to the synthesis of several of these metabolites. The development of new methodology is guided by the biosynthetic pathway proposed for the formation of the tyrosine metabolites. As an extension of this project, we recently initiated research into methods for the facile synthesis of unsymmetric biaryl ethers.
Selected Publications
″Direct capture, inhibition and crystal structure of HsaD (Rv3569c) from M. tuberculosis,″ S Barelier, R. Avellan, G. R. Gnawali, P. Fourquet, V. Roig-Zamboni, I. Poncin, V. Point, Y. Bourne, S. Audebert, L. Camoin, C. D. Spilling, S. Canaan, J-F. Cavalier and G. Sulzenbacher, FEBS J. 2023, 290, 1563.
″Synthesis and Biological Characterization of Fluorescent Cyclipostins and Cyclophostin Analogues: New Insights for the Diagnosis of Mycobacterial-Related Diseases,″ M. Sarrazin, B. P. Martin, R. Avellan, G. R. Gnawali, I. Poncin, H. Le Guenno, C. D. Spilling, J-F. Cavalier and S. Canaan, ACS Inf. Dis., 2022, 8, 2564.
″Lipolytic enzymes inhibitors: A new way for antibacterial drugs discovery,″ J-F. Cavalier, C. D. Spilling, T. Durand, L. Camoin and S Canaan, Eur. J. Med. Chem. 2021, 209, 112908.
″Synthesis of Phosphonomethyl Tetrahydrofurans via the Mori-Tamaru Reaction of Phosphonodienes,″ R. R. Paudel, J. N. Ridenour, N. P. Rath and C. D. Spilling, Org. Lett., 2020, 22, 3830
″Leaving Group Effects in a Series of Electrosprayed CcHhN1 Anthracene Derivatives″, M. T. Abutokaikah, G. R. Gnawali, J. W. Frye, C. M. Stump, J. Tschampel, M. J. Murphy, E. S. Lachance, S. Guan, C. D. Spilling and B. J. Bythell, J. Am. Soc. Mass Spec. 2019 30, 2306.
″The chemistry and biology of cyclophostin, the cyclipostins and related compounds″ C. D. Spilling, Molecules 2019, 24, 2579
″Cyclipostins and Cyclophostin analogs are multi-target inhibitors that impair growth of Mycobacterium abscessus,″ A. Madani, J. N. Ridenour, B. P. Martin, R. R. Paudel, A, A, Basir, V. Le Moigne, J-L. Herrmann, S. Audebert, L. Camoin, L. Kremer, C. D. Spilling, S. Canaan and J-F. Cavalier, ACS Infect. Dis. 2019, 5, 1597.
″Synthesis of Phostones via the Palladium-Catalyzed Ring Opening of Epoxy Vinyl Phosphonates,″ G. R Gnawali, N. P. Rath and C. D. Spilling, J. Org. Chem. 2019, 84, 8724.
"Cyclipostins and cyclophostin analogs inhibit the antigen 85C from Mycobacterium tuberculosis both in vitro and in vivo," A. Viljoen, M. Richard, P. C. Nguyen, P. Fourquet, L. Camoin, R. R. Paudal, G. R. Gnawali, C. D. Spilling, J-F. Cavalier, S. Canaan, M. Blaise and L. Kremer, J. Biol. Chem. 2018, 293, 2755.
"Cyclophostin and Cyclipostins analogues, new promising molecules to treat mycobacterial-related diseases," P. C. Nguyen, A. Madani, P. Santucci, B. P. Martin, R. R. Paudel, S. Delattre, J-L. Herrmann, C. D. Spilling, L. Kremer, S. Canaan and J-F. Cavalier, Int. J. Antimicrobial Agents, 2018, 51, 651.
″A Practical Gram-Scale Synthesis of Acrylohydroxamic Acid,″ B. C. Hamper, B. T. Sullivan, N. P. Rath and C. D. Spilling, Synthesis, 2017, 49, 5335.
″Cyclipostins and Cyclophostin analogs as promising compounds in the fight against tuberculosis,″ P. C. Nguyen, V. Delorme, A. Benarouche, B. P. Martin, R. R. Paudel, G. R. Gnawali, A. Madani, R. Puppo, V. Landry, L. Kremer, P. Brodin, C. D. Spilling, J-F. Cavalier and S. Canaan. Scientific Reports, 2017, 7, 1.
″Enantioseparation of α-Hydroxyallylphosphonates and Phosphonoallylic Carbonate Derivatives on Chiral Stationary Phases Using Sequential UV, Polarimetric, and Refractive Index Detection,″ B. C. Hamper, M. P. Mannino, M. E. Mueller, L. T. Harrison and C. D. Spilling, Chirality, 2016, 28, 656.
"Trapping hemiacetals with phosphono substituted palladium π-allyl complexes for the synthesis of substituted cyclic ethers", N: Sutivisedsak, S. Dawadi and C. D. Spilling, Tetrahedron Lett. 2015, 56, 3534.
Synthesis and comparison of the biological activity of monocyclic phosphonate, difluorophosphonate and phosphate analogs of the natural AChE inhibitor cyclophostin,″ B. P. Martin, E. Vasilieva, C. M. Dupureur and C. D. Spilling, Bioorg. & Med. Chem. 2015, 23, 7529.