-
About Us
Keith J. Stine, Chair
Mission Statement
Degree Offerings
Department Photos
Outreach Activities
-
Undergraduate Studies
Undergraduate Degrees
Undergraduate Program Advising
Undergraduate Scholarships and Awards
Research for Undergraduates
Resources
-
Graduate Studies
Graduate Program Overview
Graduate Degrees
Application to Graduate Program
Graduate Awards
Graduate Program Contact Information
-
Faculty
Faculty Contact Information
Inorganic
Organic
Analytical/Physical
Biochemistry
Chemistry Faculty Awards
-
Staff
-
Seminar Programs
Archive
Graduate Student Seminars
Robert W. Murray Lecture
Distinguished Alumni Lecture
-
Departmental News
News Archive
-
Facilities
High Field NMR Facility
MIST Lab
X-Ray Diffraction Facility
-
Alumni Interests
UMSL Chemists
Important Dates
Distinguished Alumni
Alumni Lecturers
-
Contact Information
|
|
Dr. Bauer received his Diploma (M.Sc.thesis degree) and his Ph.D from the University of Erlangen-Nuremberg. He held a postdoctoral position at the University of California, Riverside and a position as Visiting Assistant Professor prior to joining the UMSL faculty in Fall 2006. He is currently serving as Director of Graduate Studies. |
bauere@umsl.edu Office B 316E |
Research Interests
Dr. Bauer’s research interests are in the area of Organic and Organometallic Chemistry. Organometallic chemistry is the study of compounds having metal-carbon bonds. Organometallic compounds often have unique geometries and exhibit reactivities as a result of the electronic properties of the metal. Organometallic compounds are important in catalysis, medicine, and the construction of molecular scale devices (nanoscience).
Phopshoramidite and phosphinooxazoline ligands have recently attracted considerable interest as ligands for a variety of transition metal catalyzed organic transformations. These ligands are easy to synthesize and can be sterically and electronically modified at several positions in their molecular framework.
Dr. Bauer designed and synthesized several novel, electronically and sterically fine-tuned phosphoramidite and phosphinooxazoline ligands. As a new class of ligands, thio derivatives of phosphoramidites were synthesized. These ligands were subsequently converted to a variety of ruthenium, rhodium, iridium and iron complexes. The impact of the ligand structure on the physical and chemical properties of its respective metal complexes was investigated.
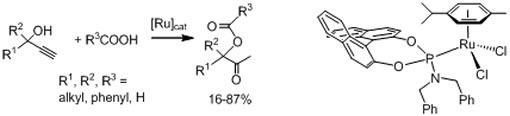
The Bauer group has shown that phosphoramidite containing half sandwich complexes of ruthenium are catalytically active in the formation of b-oxo esters from propargylic alcohols and carboxylic acids (see graphics). The ligand structure has a profound impact on the catalytic activity of the corresponding metal complex. Structurally related chiral at metal ruthenium complexes have exhibited catalytic activity in the Mukaiyama aldol reaction.
Iron is a cheap and non-toxic alternative to well-established, catalytically active transition metals. The Bauer group has demonstrated for the first time that phosphinooxazoline complexes of iron are catalytically active in the oxidation of benzylic methylene groups to ketones utilizing t-BuOOH as the oxidant.
Allenylidene complexes are cumulene-type compounds, which are readily accessible from propargylic alcohols and appropriate precursor metal complexes. The allenylidene complexes are of interest as possible intermediate in catalytic propargylic substitution reactions. Dr. Bauer has also demonstrated a route to chiral at metal allenylidene complexes, which were obtained from corresponding precursors with chirality transfer. Catalytic investigations are currently underway.
Selected Publications
″Ferrocenophanium Stability and Catalysis,″ S. A. Bezawada, N. Usto, C. Wilke, M. Barnes, R. Jagan and E. B. Bauer, Molecules, 2023, 28, 2729."Ferrocenium Ions as Catalysts: Decomposition Studies and Counteranion Influence on Catalytic Activity," K. Jurkowski and E. B. Bauer, SYNTHESIS, 2021, 53. 2007.
"Ferrocenium complex aided O-glycosylation of glycosyl halides," D. S. Talasila and E. B. Bauer. RSC Advances 2021, 11, 36814.
″Transition Metal Catalyzed Glycosylation Reactions - An Overview,″ E. B. Bauer, Org. Biomol. Chem. 2020, 18, 9160.
″Ferrocenium Cations as Catalysts for the Etherification of Cyclopropyl-Substituted Propargylic Alcohols: Ene-yne Formation and Mechanistic Insights,″ D. S. Talasila, M. J. Queensen, M. Barnes-Flaspoler, K. Jurkowski, E. Stephenson, J. M. Rabus, and E. B. Bauer, Eur. J. Org. Chem. 2019, 44, 7348.
″Cationic ruthenium complex of the formula [RuCl(2,6-diacetylpyridine)(PPh3)2]BArF and its catalytic activity in the formation of enol esters,″ M. J. Stark, D. T. Tang, N. P. Rath and E. B. Bauer, Eike, Tetrahedron Lett., 2018, 59, 873.
″Ruthenium Complexes of the general formula [RuCl2(PHOX)2] as procatalysts in propargylic substitution rections,″ N. Jourabchian, K. Jurkowski and E. B. Bauer, Catal. Comm. 2018, 106, 92.
″Recent Advances in Iron Catalyzed Oxidation Reactions of Organic Compounds,″ E. B. Bauer, Israel J. Chem. 2017, 57, 1131.
"Synthesis, structural characterization and catalytic activity of indenyl complexes of ruthenium bearing fluorinated phosphine ligands,″ M. J. Stark, M. J. Shaw, A. Fadamin, N. P. Rath and E. B. Bauer, J. Organomet. Chem. 2017, 847, 41.
″Spectroscopic investigation and direct comparison of the reactivities of iron pyridyl oxidation catalysts,″ Y. Song, H. G. Mayes, M. J. Queensen, E. B. Bauer and C. M. Dupureur, Spectrochimica Acta, 2017, 174, 130.
″Synthesis,Characterization and Catalytic Activity of Indenyl Tris(N-pyrrolyl-phosphine Complexes of Ruthenium,″ M. J. Stark, M. J. Shaw, N. P. Rath and E. B. Bauer, Eur. J. Inorg. Chem. 2016, 1093.102.
″Ferrocenium Hexafluorophosphate as an inexpensive mild catalyst for the etherification of Propargylic alcohols,″ M. J. Queensen, J. M. Rabus and E. B. Bauer, J. Mol. Catal. A, 2015, 407, 221.
″Iron Catalysis: Historic Overview and Current Trends, ″ E. B. Bauer, Topics in Organometallic Chemistry, 2015, 50, 1
″Topics in Organometallic Chemistry, Vol 50, Iron Catalysis II,″ E. B. Bauer, Ed.; Springer, NY, 2015, 262 pp.
"Diastereoselective Attack on Chiral-at-Metal Ruthenium Allenylidene Complexes to Give Alkynyl Complexes" M. Queensen, N. P. Rath, and E. B. Bauer, Organometallics, 2014, 33, 5052.
