A Protocol for Deriving Values for D fusHm(298.15 K) and D vapHm(298.15 K).
Applications in Obtaining D subHm (298.15 K)
James Chickos, Gary Nichols, Joe Wilson,
Jennifer Orf, Paul Webb and Jin Wang
Department of Chemistry
University of Missouri-St. Louis
St. Louis MO USA 63121
ABSTRACT. An indirect method for obtaining sublimation enthalpies is described. The method consists of combining experimental or estimated fusion enthalpies adjusted to 298.15 K with either vaporization enthalpies measured at elevated temperatures and adjusted for temperature or obtained directly at 298.15 K by correlation gas chromatography. Some relationships used to adjust phase change enthalpies with temperature are discussed and their use is demonstrated on a series of compounds. These equations are used to adjust the fusion enthalpies of hydrocarbons to 298.15 K. The corresponding fusion entropies at 298.15 K are parameterized using group additivity relationships and the resulting group values are used to estimate fusion entropies and enthalpies at 298.15 K. The techniques discussed are applied to the evaluation of the sublimation enthalpies of perylene and coronene.
1 Introduction
Sublimation enthalpies are an important macroscopic measure of the magnitude of intermolecular interactions in the solid state. Measurement of this quantity serves a variety of useful purposes. This includes uses in thermochemistry as a means of converting condensed phase enthalpies of formation to the gas phase [1], quantifying mass transport by establishing the relationship between vapor pressure and temperature [2], and as an experimental measure of the cumulative effect of the intermolecular forces that lead to the packing observed in the solid state of crystalline materials [3].
A variety of experimental techniques have been developed and applied to directly measure the sublimation enthalpies of solids [4]. Sublimation enthalpies of many other materials cannot be measured directly either because of low volatility or chemical instability. Our recent interests have been focused on the development of methods for obtaining hypothetical phase change enthalpies and entropies that retain their thermodynamic relevance but are measured or derived indirectly. Our attention has focused on experimental as well as estimation techniques.
Sublimation enthalpies have been obtained indirectly by using Eqn. 1. This equation has been used infrequently because vaporization enthalpy data on solids at 298.15 K are not usually available and extrapolations of vaporization enthalpies from measurements performed at elevated temperatures to 298.15 K can be problematic. In addition, Eqn. (1), as written is an approximation. A thermodynamic equality results only
D
subHm (298.15 K) » DvapHm(298.15 K) + DfusHm(Tfus) (1)D
subHm (298.15 K) = DvapHm(298.15 K) + DfusHm(298.15 K) (2) DsubHm (298.15 K) = DvapHm(298.15 K) + ![]() (3)
(3)
when all three enthalpies are referenced to the same temperature (Eqn. 2), 298.15 K in this case. Eqn. 3 provides a more accurate description in so far as the total phase change enthalpy of the solid, ![]() , also includes the enthalpies of all phase transitions occurring between the melting point, Tfus and 298.15 K.
, also includes the enthalpies of all phase transitions occurring between the melting point, Tfus and 298.15 K.
A protocol that adjusts vaporization, sublimation and fusion enthalpies with temperature has been reported recently [5-7]. The use of this protocol is discussed below. Similarly, sublimation enthalpies are generated by combining vaporization enthalpies with experimental fusion enthalpies. The vaporization enthalpies are obtained from the literature or measured indirectly by correlation gas chromatography. Both vaporization and fusion enthalpies adjusted to 298.15 K are used. The results are compared to experimental measurements and evaluated on this basis. In addition, sublimation enthalpy measurements reported on two compounds, perylene and coronene are discussed. The experimental measurements on these two compounds illustrate the difficulties encountered when dealing with high melting samples of low volatility. These physical properties identify an important limitation of the protocol discussed and highlight the useful role that estimation techniques can play. The physical properties of both coronene and perylene have been the subject of recent theoretical treatments [8, 64].
2 Background
2.1. TEMPERATURE ADJUSTMENTS OF DsubHm AND DvapHm
Sublimation enthalpies are very frequently measured at temperatures other than 298.15 K. The temperature of measurement is usually determined by the volatility of the sample of interest. A variety of protocols have been developed to adjust sublimation enthalpies measured at temperature Tm, where Tm represents either a single temperature or the mean of a range of temperatures, back to 298.15 K. The thermodynamic cycle used to adjust sublimation enthalpies from Tm to 298.15 K is illustrated in Figure 1.
D subHm(Tm)
Figure 1. Thermodynamic cycle for adjusting sublimation enthalpies to 298.15 K.
If the heat capacities of the gas and solid phase are known, Cpc and Cpg, respectively, then the sublimation enthalpy at 298.15 K can be related to experimental measurements by equation 4. This equation, generally referred to as Kirchhoff’s equation, can be used to adjust sublimation enthalpy measurements to any reference temperature. Treating the heat capacities of the two phases as independent of temperature and integrating results in equation 5.
D
subHm (298.15 K) = DsubHm(Tm) +D
subHm (298.15 K) = DsubHm(Tm) + (Cpc - Cpg) [Tm-298.15] (5)A diagram similar to Figure 1 (not shown) can also be used to adjust vaporization enthalpies measured at temperature Tm,
DvapHm(Tm), to 298.15 K. Replacing the sublimation enthalpy terms with the corresponding vaporization enthalpy and substituting the heat capacity of the liquid, Cpl, for that of the solid, results in Kirchhoff’s equation for liquids, equations 6 and 7.D
D
vapHm (298.15 K) = DvapHm(Tm) + (Cpl - Cpg) [Tm-298.15] (7)Difficulties associated in using equations 5 and 7 are generally related to the unavailability of an experimental heat capacity, usually for the gas phase and in some instances for the condensed phases as well. Experimental gas phase heat capacities for substances that are solids or liquids at 298.15 K are generally not available. Gas phase heat capacities can be estimated from group additivity methods or calculated from statistical mechanics [9, 10]. Condensed phase heat capacities can be estimated by group methods [11, 12]. In addition, various approximations have been developed. A brief summary of the various methods that have been used in place of equations 5 and 7 are summarized in the Tables 1 and 2.
Table 1. Equations for Temperature Adjustments of Vaporization Enthalpies
|
Vaporization Enthalpies |
Equation |
[ref.] |
|
(Cpl - Cpg) [Tm-298.15] = 54.4[Tm-298.15] |
8 |
[13] |
|
(Cpl - Cpg) [Tm-298.15] = 50.2[Tm-298.15] |
9 |
[14] |
|
(Cpl - Cpg) [Tm-298.15] = [10.58 + 0.26Cpl][Tm-298.15] |
10 |
[15] |
Table 2. Equations for Temperature Adjustments of Sublimation Enthalpies
|
Sublimation Enthalpies, J mol-1 |
Equation |
[ref.] |
|
(Cpc - Cpg) [Tm-298.15] = 2R[Tm-298.15] |
11 |
[4] |
|
(Cpc - Cpg) [Tm-298.15] = 6R[Tm-298.15] |
12 |
[16] |
|
(Cpc - Cpg) [Tm-298.15] = 40[Tm-298.15] |
13 |
[17] |
|
(Cpc - Cpg) [Tm-298.15] = 60[Tm-298.15] |
14 |
[18] |
|
(Cpc - Cpg) [Tm-298.15] = [0.75 + 0.15Cpc(298.15 K)][Tm-298.15] |
15 |
[15] |
A major limitation of most of the equations listed in Tables 1 and 2 is that the heat capacity adjustments are treated as universal constants independent of molecular structure. Only equations 10 and 15 are sensitive to differences in molecular structure. These two equations were derived from experimental data that strongly suggested that differences in heat capacities between the condensed and gas phases are dependent both on molecular architecture and size [15]. Heat capacities of the solid or liquid phase are required when using equations 10 and 15; experimental or estimated values can be used.
2.2. A TEST OF TWO HEAT CAPACITY EQUATIONS
Critically evaluated experimental vaporization enthalpies measured at a variety of temperatures generally by calorimetric methods are readily available [19]. The use of equations 7 and 10 in adjusting experimental enthalpies to 298.15 K is illustrated and compared in Table 3. The second column of the table represents the experimental vaporization enthalpy measured at temperature, Tm. The estimated heat capacities of the liquid and gas phase (when available) at 298.15 K are included in the fourth and fifth columns. The heat capacity of the gas and liquid phases were estimated using group additivity methods developed by Benson [10] and Chickos et al. [11], respectively. The adjusted vaporization enthalpies at 298.15 K using equations 7 and 10 are listed in the fifth and sixth columns, respectively. The last column reports the experimental value measured at 298.15 K. Assuming no error in the experimental vaporization enthalpies, the average absolute error between calculated and experimental
DvapHm (298.15K) using equation 7 and 10 is 1.9% and 1.7 %, respectively. Both equations give equally good results with this limited data set.Table 3. Adjustment of Vaporization Enthalpies with Temperature
|
D vapHm (Tm)a |
Tm (K) |
Cp g Cpl |
D vapHm (298.15K)estimated Eqn. 7 Eqn. 10 |
D vapHm (298.15K)a |
|
C 4H6ClF3O 2-chloro-1,1,2-trifluoroethyl ether |
32040 |
368 |
na b |
212 |
36627 |
37500 |
|
|
C 4H10S2 diethyl disulfide |
39250 |
400 |
142 |
208 |
46024 |
45806 |
45170 |
|
C 5H10O2 propyl ethanoate |
33940 |
375 |
na b |
197 |
38654 |
39830 |
|
|
C 6H5Cl chlorobenzene |
34850 |
405 |
98.7 |
155 |
40866 |
40250 |
40970 |
|
C 6H6S benzenethiol |
41230 |
417 |
106 |
173 |
49279 |
47800 |
47490 |
|
C 6H12O 3-hexanone |
35350 |
397 |
150 |
217 |
41940 |
41921 |
42450 |
|
C 7H8O anisole |
38880 |
427 |
na b |
219 |
47519 |
46840 |
|
|
C 8H10 p-xylene |
33770 |
440 |
128 |
188 |
42143 |
42115 |
42370 |
|
C 12H26O 1-dodecanol |
84670 |
343 |
295 |
375 |
88284 |
89526 |
91960 |
a
reference [19]; bnot available.Demonstration of the applicability of equation 15 is more problematic. Temperature adjustments of sublimation enthalpies are smaller than for vaporization enthalpies and the uncertainties associated with many sublimation enthalpies are as large or larger than the adjustment. Consequently, temperature adjustments are barely if ever perceptible above the "noise level" associated with repetitive measurements. This is illustrated in Table 4 for anthracene, a substance whose sublimation enthalpy has be reported numerous times since 1950 and which has been used as a material used to test sublimation enthalpy apparatus [20, 21]. The mean sublimation enthalpy for anthracene from these measurements is 100721 ± 4122 or 100234 ± 4009 J mol-1 depending on whether equation 15 or 5 is used for the temperature adjustment (see Table 5 for Cp values used). The average temperature adjustment is roughly half the uncertainty. As a consequence of this uncertainty, and the fact that heat capacities are not always available, temperature adjustments to sublimation enthalpies have often been ignored. We would like to demonstrate that inclusion of these adjustments, even though small, is important and can lead to significant improvements in accuracy especially when sublimation
Table 4. Sublimation Enthalpies of Anthracene.
|
D subHm(Tm/K)J mol-1 |
Tm/K |
(Cpc-Cpg) D T , J mol-1 |
(0.75+0.15Cpc) D T, J mol-1 |
D subHm(298.15 K), J mol-1Eqn. 5 Eqn. 15 |
[Ref.] |
|
99700 |
383 |
1968 |
2723 |
101668 |
102423 |
[22] |
|
98745 |
346 |
1102 |
1524 |
99847 |
100269 |
[23] |
|
102600 ± 1300 |
338 |
924 |
1279 |
103524 |
103879 |
[20] |
|
94592 |
376 |
1805 |
2499 |
96398 |
97091 |
[24] |
|
91800 ± 3766 |
303 |
112 |
156 |
91912 |
91956 |
[25] |
|
104500 ± 1000 |
298 |
0 |
0 |
104500 |
104500 |
[26] |
|
104766 ± 800 |
383 |
1971 |
2728 |
106737 |
107494 |
[27] |
|
97194 ± 1674 |
351 |
1229 |
1701 |
98423 |
98895 |
[28] |
|
97069 ± 837 |
298 |
0 |
0 |
97069 |
97069 |
[21] |
|
95814 ± 5858 |
303 |
112 |
156 |
95926 |
95969 |
[29] |
|
101035 ± 460 |
393 |
2188 |
3028 |
103223 |
104063 |
[30] |
|
99690 ± 750 |
393 |
2200 |
3044 |
101890 |
102734 |
[30] |
|
98300 ± 560 |
351 |
1214 |
1680 |
99514 |
99980 |
[31] |
|
100834 |
388 |
2084 |
2884 |
102918 |
103718 |
[32] |
|
103400 |
338 |
924 |
1279 |
104324 |
104679 |
[33] |
|
97487 ± 2090 |
399 |
2331 |
3225 |
99818 |
100713 |
[34] |
|
92268 ± 8786 |
364.15 |
1121 |
2118 |
93799 |
94386 |
[35] |
|
101600 ± 2050 |
347 |
1531 |
1552 |
102721 |
103152 |
[33] |
enthalpies are evaluated indirectly.
Sublimation enthalpies at 298.15 K can be obtained indirectly by examining the thermochemical cycle illustrated in Figure 2. According to this figure, combining the vaporization enthalpy and fusion enthalpies at 298.15 K also results in
DsubHm(298.15 K). Vaporization enthalpies are available at 298.15 K or can be adjusted to this temperature using equation 10. Since fusion enthalpies are usually measured at either Tfus or at the triple point, it becomes necessary to adjust this enthalpy to 298.15 K. To achieve this result the heat capacities of the solid and liquid phase are necessary. A description ofFigure 2. Thermodynamic cycle for relating phase change enthalpies with temperature.
how fusion enthalpies can be adjusted to 298.15 K by combining equations 10 and 15 follows.
The fusion enthalpy at 298.15 K can be evaluated from the equilibrium value measured at Tfus by correcting for the heat capacity differences between the liquid and solid phases as given by equation 16. It is also necessary to include the enthalpies associated with any additional phase changes occurring between Tfus and 298.15 K (
DpceHm(Tpc ). As noted above, this is referred to as the solid-liquid phase change enthalpy at 298.15 K (DslpceHm(298.15 K)). Treating the heat capacities of the liquid and solid as independent of temperature, results in equation 17. Alternatively, subtracting equation 10 from equation 15 results in an equivalent relationship, equation 18. A comparison of the results obtained in using equations 17 and 18 are provided in Table 4 for a variety of organic compounds.D
lpceHm(298.15 K) = DfusHm(Tfus) + DpceHm(Tpc ) +D
tpceHm(298.15 K) = DfusHm(Tfus) + DpceHm(Tpc ) + (Cpl - Cpc)(Tfus-298.15) 17D
tpceHm(298.15 K) = DfusHm(Tfus) + DpceHm(Tpc ) +[(0.75 + 0.15 Cpc) – (10.58 + 0.26 Cpl)](Tfus - 298.15) 18
The heat capacities for the solid and liquid phases were estimated as previously described [11]. Heat capacities for the gas phase were estimated using the method described by Benson [10] and as modified by Domalski and Hearing [9]. The lack of necessary group values to estimate the gas phase heat capacities of compounds such as benzoic acid, benzophenone and 2,4-nitrotoluene limits the usefulness of equations 5 and 7. Columns 2 and 3 in Table 5 contain the experimental fusion enthalpy and fusion
Table 5. A Comparison of Equations 17 and 18 in Adjusting Sublimation Enthalpies with Temperature
|
Compound |
D fusH(lit)a |
T fus |
D vapH (Tm)(lit) b |
T m |
Cp l |
Cp c |
Cp g |
D subHm(298) Eqn. 7,17 |
D subHm(298) Eqn. 10,18 |
D subHm(298) (lit) |
|
anthracene |
29372 |
489 |
59390 c |
519 |
279 |
209 |
186 |
95778 |
97382 |
100721 d |
|
benzoic acid |
18006 |
396 |
69189 x |
396 |
212 |
149 |
na w |
na w |
89478 |
89700 e |
|
benzophenone |
18194 |
321 |
73462 f |
353 |
300 |
220 |
na w |
na w |
95263 |
95570 f |
|
biphenyl |
18580 |
342 |
61762 g |
340 |
249 |
192 |
161 |
81528 |
81478 |
82030 p |
|
2,4-dinitrotoluene |
20120 h |
343 |
76862 |
359 |
263 |
227 |
na w |
na w |
99806 |
99600 I |
|
2-isopropyl-5-methylphenol |
22010 j |
324 |
58442 |
396 |
291 |
220 |
205 |
87057 |
87544 |
89264 I |
|
naphthalene |
19046 |
353 |
50246 k |
371 |
205 |
157 |
134 |
71808 |
71727 |
72600 l |
|
1,8-octanediol |
36100 h |
333 |
101100 m |
356 |
361 |
262 |
195 |
143373 |
141001 |
139300 n |
|
phenanthrene |
16681 a,o |
372 |
71210 o |
372 |
279 |
209 |
186 |
89576 |
90241 |
90880 p |
|
phenol |
11514 |
314 |
57375 |
329 |
177 |
119 |
102 |
70290 |
70032 |
69700 q |
|
pyrene |
17355 r |
424 |
75640 s |
433 |
295 |
218 |
203 |
95775 |
97998 |
100200 t |
|
trans stilbene |
27690 |
397 |
65460 |
434 |
304 |
235 |
203 |
100000 |
100018 |
102400 p |
|
1,2,4,5-tetra-chlorobenzene |
26340 |
421 |
52046 |
434 |
228 |
184 |
151 |
83417 |
82773 |
83200 u |
|
1,3,5-triphenyl-benzene |
22930v |
449 |
116570v |
477 |
484 |
366 |
327 |
149861 |
151756 |
149499 p |
a
reference [36] unless otherwise noted; breference [37] unless otherwise noted; creference [38]; dsee text; ereference [40]; freference [41]; greference [39]; hreference [43]; ireference [4]; jreference [44]; kreference [45]; lreference [62]; mreference [47]; nreference [48]; oreference [49]; preference [58]; qreference [50]; rreference [51]; sreference [52]; treference [1]; ureference [53]; vreference [54]; wnot available; xreference [56].temperature, while the experimental vaporization enthalpies measured at the mean temperature, Tm, are included as columns 4 and 5. The sublimation enthalpies, adjusted with the aid of equations 7 and 17 and 10 and 18, are included in columns 9 and 10, respectively. A comparison of the standard deviation observed in differences between experimental and estimated values for the entries in Table 5, columns 11 and 9 and 11 and 10, results in standard deviations of ± 2527 and ± 1589 J mol-1 respectively. Both methods give good results. While the number of entries in this table is limited, the use of equation 18 appears to be slightly more accurate in this comparison and more flexible
with regards to its applicability. The use of equations 10, 15 and 18 do not require the heat capacity of the gas phase and this can be an additional advantage if the appropriate group values are not available.
3 Vaporization Enthalpies of Solids at 298.15 K
3.1. APPLICATIONS OF CORRELATION GAS CHROMATOGRAPHY
Correlation gas chromatography has been used to measure the vaporization enthalpies of a large number of organic compounds for which there already exists a reliable database of structurally related materials with known vaporization enthalpies. We have found that the vaporization enthalpies of the n-alkanes serve as suitable standards for hydrocarbons of any structure. The procedure has been detailed previously [55, 6, 7]. A brief outline is provided below using the data in Table 6 as an example. The retention time of carbon tetrachloride as a function of temperature is shown as the first entry. Unlike the other
Table 6. Gas Chromatographic Retention Times
|
Temperature (K) |
543.15 |
553.15 |
563.15 |
573.15 |
583.15 |
|
Compound |
Retention (s) |
Time |
|||
|
carbon tetrachloride |
46.5 |
47.58 |
48.36 |
48.18 |
48.48 |
|
octadecane |
67.62 |
64.98 |
62.7 |
60 |
58.38 |
|
eicosane |
82.2 |
76.32 |
71.64 |
66.9 |
63.9 |
|
docosane |
105.9 |
94.38 |
85.5 |
77.46 |
72.12 |
|
tetracosane |
144.42 |
123.06 |
106.98 |
93.54 |
84.36 |
|
octacosane |
306.9 |
239.52 |
191.34 |
154.5 |
129.42 |
|
perylene |
348.9 |
283.02 |
232.92 |
192 |
162.9 |
|
coronene |
1312.98 |
984.54 |
748.74 |
572.1 |
448.86 |
 compounds, the retention time of carbon tetrachloride is generally observed to increase with increasing temperature. This is characteristic of any material which is not significantly retained on the column and this behavior parallels the increase in viscosity of the carrier gas with temperature which is helium in this case. The retention time of carbon tetrachloride is therefor used to correct for the dead volume of the column. The difference in retention time between each substance and carbon tetrachloride is due to the residence time of the substance on the column. This residence time is inversely proportional to the vapor pressure of the substance "dissolved" in the stationary phase of the column. Furthermore, since the "equilibrium or steady state" established, is between the vapor and "solution", the observed vapor pressure is independent of whether the
compounds, the retention time of carbon tetrachloride is generally observed to increase with increasing temperature. This is characteristic of any material which is not significantly retained on the column and this behavior parallels the increase in viscosity of the carrier gas with temperature which is helium in this case. The retention time of carbon tetrachloride is therefor used to correct for the dead volume of the column. The difference in retention time between each substance and carbon tetrachloride is due to the residence time of the substance on the column. This residence time is inversely proportional to the vapor pressure of the substance "dissolved" in the stationary phase of the column. Furthermore, since the "equilibrium or steady state" established, is between the vapor and "solution", the observed vapor pressure is independent of whether the
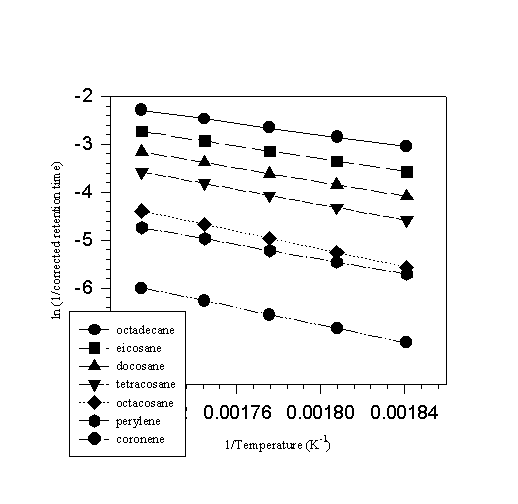
Figure 3. A plot of ln[1/rt] against 1/T (K-1).
solute is a solid or a liquid. A Clausius Clapeyron plot of the natural logarithm of 1/(corrected retention or residence time (rtc)) as a function of 1/temperature (K-1), results in a straight line whose slope affords the enthalpy of transfer from solution to the vapor divided by the gas constant. We have found that if the standards are chosen carefully, the enthalpy of transfer from solution to the vapor, ![]() , correlates with the vaporization enthalpies of the standards, regardless of whether the compounds are liquids or solids [6]. Figure 3 illustrates the type of correlation typically observed in ln(1/rtc) versus 1/T plots. Enthalpies of transfer,
, correlates with the vaporization enthalpies of the standards, regardless of whether the compounds are liquids or solids [6]. Figure 3 illustrates the type of correlation typically observed in ln(1/rtc) versus 1/T plots. Enthalpies of transfer, ![]() , are listed in the second column of Table 7. A second correlation between
, are listed in the second column of Table 7. A second correlation between ![]() and D
vapHm(298.15 K) results in Figure 4 and a least squares
and D
vapHm(298.15 K) results in Figure 4 and a least squares
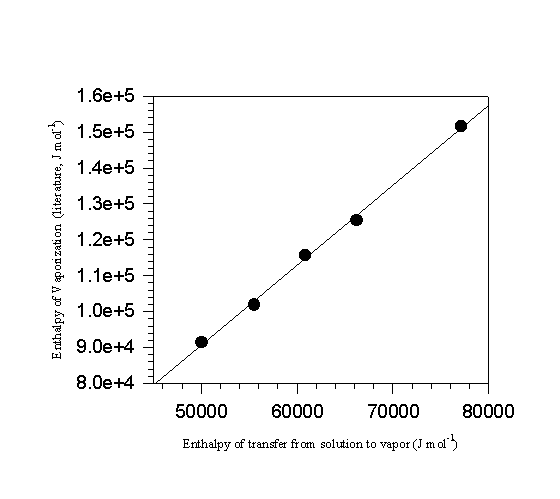 Figure 4. A plot of enthalpy of vaporization against the enthalpy of transfer from solution to the vapor of the standards.
Figure 4. A plot of enthalpy of vaporization against the enthalpy of transfer from solution to the vapor of the standards.
linear regression of the data produces equation 19. This equation is then used to calculate the vaporization enthalpies of each of the compounds included in the correlation, the fifth column in the table.
Table 7. Vaporization Enthalpies by Correlation Gas Chromatography
|
Compound |
|
Correlation Coefficient r 2 |
D vapHm(298.15 K)lit a |
D vapHm(298.15 K)eqn 19 |
|
octadecane |
50088 ± 322 |
0.9999 |
91400 |
90696 ± 2730 |
|
eicosane |
55504 ± 423 |
0.9998 |
101800 |
102755 ± 3030 |
|
docosane |
60876 ± 416 |
0.9999 |
115600 |
114720 ± 3320 |
|
tetracosane |
66289 ± 367 |
0.9999 |
125600 |
126772 ± 3610 |
|
octacosane |
77104 ± 330 |
0.9999 |
151400 |
150857 ± 4200 |
|
perylene |
64164 ± 438 |
0.9999 |
122040 ± 3500 |
|
|
coronene |
75966 ± 265 |
0.9999 |
148323 ± 4140 |
a
reference [7]D
vapHm(298.15 K) = 2.233.2. VAPORIZATION ENTHALPIES OF SOLIDS BY C-GC
The protocol just described has been applied to a group of hydrocarbons and to a few hydrocarbon derivatives, all of which are solids at room temperature. The results are summarized in Table 8. Vaporization enthalpies of these materials have been measured above their melting point. The vaporization enthalpies of these materials have been adjusted to 298.15 using both equations 7 and 10 and are listed in columns 6 and 7 of the table. Vaporization enthalpies measured by correlation-gas chromatography are
Table 8. A Comparison of Vaporization Enthalpies Obtained by Gas Chromatography with Literature Values.
|
D vapHm(Tm)[lit.] |
Tm (K) |
Cpl |
Cpg |
D vapHm(298 K)Eqn. 7 |
D vapHm(298 K)Eqn. 10 |
D vapHm(298 K)c-gc |
|
|
anthracene |
59390 a |
519 |
279 |
219 |
72796 |
77760 |
79812 |
|
biphenyl |
61762 b |
340 |
249 |
181 |
64573 |
64910 |
66244 |
|
naphthalene |
50246 c |
371 |
205 |
151 |
55415 |
54868 |
53438 |
|
b -naphthol |
59697 d |
416 |
252 |
154 |
71163 |
68653 |
77116 |
|
phenanthrene |
71210 e |
372 |
279 |
210 |
76298 |
77325 |
78650 |
|
trans stilbene |
65458 f |
434 |
304 |
243 |
73772 |
77640 |
79725 |
|
thymol |
58442 f |
396 |
291 |
194 |
67981 |
66893 |
71176 |
|
1,3,5-triphenylbenzene |
116570 g |
477 |
484 |
327 |
144676 |
140969 |
139950 |
a
reference [38]; breference [39]; creference [45]; dreference [39]; ereference [36, 49]; freference [37]; greference [54].listed in the last column of the table. A comparison of the last three columns shows that the c-gc results are in good agreement with the experimental values once the experimental values are adjusted for temperature. Standard deviations of the differences between the c-gc results and equations 7 and 10 are ± 3927 and ±
3132 J mol-1, respectively. In this case, there is no basis for identifying which equation provides the best temperature adjustment. However, the c-gc results appear to correlate better with the results of Eqn. 19Vaporization enthalpies obtained by correlation gas chromatography for the solids in Table 8 along with some additional compounds are combined with temperature adjusted solid-liquid phase change enthalpies to obtain sublimation enthalpies at 298.15 K. These results are provided in Table 9. Heat capacity estimates for the gas phase are not available for some of the solids in Table 9 but can be estimated for the condensed phases.
3.3. SUBLIMATION ENTHALPIES BY DSC-CGC
The sublimation enthalpies in Table 9
have been calculated with the aid of equations 3 and 17 and 3 and 18 and are included in the last two columns. These values can be compared with sublimation enthalpies measured directly, column 7. It should be noted that experimental sublimation enthalpies from the literature available at temperatures other than 298.15 were corrected to 298.15 K using equation 15. Temperature adjustments to sublimation enthalpies are usually small as noted above. The standard deviation of the differences observed between columns 7 and 8 and 7 and 9 are ± 2654 and ± 2095 J mol-1, respectively. Regardless of the protocol used, vaporization enthalpies obtained by correlation gas chromatography and combined with temperature adjusted fusion enthalpies result in sublimation enthalpies that are in good agreement with values measured directly.3.4. SUBLIMATION ENTHALPIES OF PERYLENE AND CORONENE
There are several reports of the sublimation enthalpies of perylene and coronene in the literature. Coronene, mp 715 K, is an example of a molecule that is quite non-volatile. The results are summarized in Table 10 which include the reported sublimation enthalpies at the mean temperature of measurement and values adjusted to 298.15 K using equation 15. As indicated by the standard deviation of the mean, the
Table 9. Sublimation Enthalpies by Correlation Gas Chromatography and Differential Scanning Calorimetry
|
Cp l |
Cp c |
D fusHm(Tfus) |
T fus |
D vapHm(298)c-gc |
D subHm(298)lit. |
D subHm(298)Calcd. Eqn. 3, 17 |
D subHm(298)Calcd. Eqn. 3, 18 |
|
|
acenaphthene |
244 |
188 |
21462 a |
366 |
66210 |
85310 b |
83895 |
84617 |
|
azulene |
205 |
157 |
17530b |
374 |
58191 |
76880 b |
72079 |
72719 |
|
anthracene |
279 |
209 |
29372 a |
489 |
79812 |
100721 c |
95779 |
99436 |
|
benzoic acid |
212 |
149 |
18006 a |
396 |
78865 |
89700 d |
90744 |
92711 |
|
biphenyl |
249 |
192 |
18580 a |
342 |
66244 |
82030 b |
82341 |
82821 |
|
cyclododecane |
311 |
295 |
14800g |
334 |
63017 |
76400 h |
77258 |
76155 |
|
cyclotetradecane |
363 |
344 |
28870 i |
328 |
68459 |
92170 b |
96786 |
95763 |
|
dimethyl oxalate |
196 |
154 |
21100 k |
325 |
53600 |
75200 l |
73555 |
73680 |
|
fluorene |
262 |
199 |
19578 e |
388 |
72340 |
86130 b |
86263 |
87602 |
|
naphthalene |
205 |
157 |
19046 a |
353 |
53438 |
72600 m |
69850 |
70313 |
|
b -naphthol |
252 |
171 |
18790 a |
394 |
77116 |
94620 n |
88257 |
91178 |
|
phenanthrene |
279 |
209 |
15720 a |
374 |
78650 |
91810 b |
89042 |
90496 |
|
trans stilbene |
304 |
235 |
27690 a |
398 |
79725 |
102400 b |
100463 |
102016 |
|
succinonitrile |
159 |
138 |
3703 a |
331 |
64559 |
69803 o |
67579 |
67261 |
|
thymol |
291 |
220 |
22010 p |
342 |
71176 |
91026 o |
90062 |
90880 |
|
1,3,5-triphenylbenzene |
484 |
366 |
22928 q |
446 |
139950 |
149545 q |
145432 |
150936 |
|
triphenylmethane |
395 |
297 |
21979 a |
365 |
94552 |
112320 b |
109958 |
111981 |
a
reference [36] ; breference [7]; csee text; dreference [40]; ereference [49]; freference [39]; greference [57]; hreference [58]; ireference [44]; jreference [59]; kreference [43]; lreference [42]; mreference [61]; nreference [62]; oreference [1]; preference [44]; qreference [54];Table 10. Sublimation Enthalpies of Perylene and Coronene
|
Compound |
D subHm(Tm)J mol-1 |
T m(K) |
Cp c |
Cp l |
D vapHm(298)c-gc |
D subHm(298)Eqn. 3, 15 |
Mean |
D fusHm(Tfus) |
T fus(K) |
D subHm(298)Eqn. 3, 18 |
|
Perylene |
123200 a |
383 |
270 |
369 |
126698 |
|||||
|
145200 b |
298 |
145200 |
137564 |
|||||||
|
139000 c |
418 |
143941 |
± 8698 |
|||||||
|
129600 d |
415 |
134417 |
||||||||
|
122040 |
32580 |
551 |
138087 |
|||||||
|
Coronene |
143200 a |
383 |
288 |
401 |
146924 |
150122 |
||||
|
135900 e |
442 |
142213 |
± 6745 |
|||||||
|
151900 d |
407 |
156677 |
||||||||
|
147000 c |
473 |
154674 |
||||||||
|
148323 |
19200 |
710 |
138285 |
a
reference [22]; breference [65]; creference [66]; dreference [67]; ereference [37].sublimation enthalpies listed in the table for both of these materials are not known with a high degree of certainty. To determine whether correlation gas chromatography combined with differential scanning calorimetry could be useful in such circumstances, we have combined the vaporization enthalpies reported in Table 7 for perylene and coronene with the corresponding experimental fusion enthalpies according to the protocol just described. The pertinent data and results are included in columns 6, and 9-11 of Table 10. The gc-dsc results obtained for perylene are in good agreement with the mean value of 135151 ± 9265 J mol-1 reported in the table. The gc-dsc results obtained for coronene, however, are absurd. The sublimation enthalpy at 298.15 K is smaller than the corresponding vaporization enthalpy measured by correlation gas chromatography. An examination of the melting point of coronene reveals the cause. Equations 10 and 15 have been applied with some success up to temperatures in the neighborhood of 500 K [6, 7]. The use of these equations to adjust phase change enthalpies over larger temperatures ranges is expected to result in larger uncertainties and to fail at some point [5]. Even with perylene, mp 551 K, the uncertainty associated with the use of equations 10 and 15 is likely to be larger than for most compounds in Table 9. The limitations associated with the use of these equations at higher temperatures, prompted us to address this problem from a different perspective.
4 Estimation of Phase Change Entropies at 298.15 K
4.1. GROUP ADDITIVITY RELATIONSHIPS FOR HYDROCARBONS
The use of equation 18 to adjust experimental fusion enthalpies from the experimental melting point to 298.15 K suggested an alternative approach for dealing with materials that exhibit high melting points. We have previously reported a group additivity approach for estimating total phase change entropies and enthalpies associated in going from 0 K to the isotropic liquid at the melting point [8, 45]. This estimation protocol provides total phase change entropies and enthalpies at the melting point. Since equation 18 appears successful in adjusting fusion enthalpies below 500 K, our approach in this instance has been to first adjust the experimental total phase change enthalpies of our database to 298.15 K using this equation. The temperature adjusted total phase change enthalpies were then used to calculate total phase change entropies at 298.15 K and in turn the entropy was then parameterized as before [45]. This has been accomplished for hydrocarbons. The protocol used to estimate total phase change entropy and enthalpy of hydrocarbons at their melting point has been described recently [46]. The estimation of total phase change entropy and enthalpy at 298.15 K uses an identical protocol and will not described here in detail.
Our database used consisted of the fusion enthalpies of 253 hydrocarbons. Compounds with high melting points such as coronene were not included in the database. The parameterization resulted in the group values listed in columns 3 and 4 of Table 12A and B. The first two columns of this table summarize group values that can be used to
Table 12A. Group Contributions for Acyclic and Aromatic Hydrocarbonsa
|
Group Values (J mol-1 K-1) Aliphatic and Aromatic Carbon Groups Tfus 298.15 K GI |
|
primary sp3 |
CH 3- |
A |
17.6 |
12.7 |
||
|
secondary sp3 |
>CH 2, |
B |
7.1 |
1.31 |
6.4 |
1.48 |
|
tertiary sp3 |
-CH<, |
C |
-16.4 |
-8.2 |
||
|
quaternary sp3 |
>C<, |
D |
-34.8 |
-25.8 |
||
|
secondary sp2 |
=CH 2 |
E |
17.3 |
9.2 |
||
|
tertiary sp2 |
=CH- |
F |
5.3 |
5.5 |
||
|
quaternary sp2 |
=CR- |
G |
-10.7 |
-1.9 |
||
|
tertiary sp |
H-Cº |
H |
14.9 |
13.4 |
||
|
quaternary sp |
-Cº |
I |
-2.8 |
1.6 |
||
|
aromatic tertiary sp2 |
=C aH- |
J |
7.4 |
5.9 |
||
|
quaternary aromatic sp2 carbon |
||||||
|
adjacent to an sp3 atom |
=C aR- |
K |
-9.6 |
-4 |
||
|
peripheral quaternary aromatic sp2 |
||||||
|
carbon adjacent to an sp2 atom |
=C aR- |
L |
-7.5 |
-4.9 |
||
|
internal quaternary aromatic sp2 |
||||||
|
carbon adjacent to an sp2 atom |
=C aR- |
M |
-0.7 |
-2.2 |
Table 12B. Contributions of the Cyclic Hydrocarbon Portions of the Molecule
|
Group Values (J mol-1 K-1) Cyclic Carbon Groups Tfus 298.15 K GI GI |
|
cyclic tertiary sp3 |
>C cHR |
N |
-14.7 |
-9.9 |
||
|
cyclic quaternary sp3 |
>C cR2 |
O |
-34.6 |
22.4 |
||
|
cyclic tertiary sp2 |
=C cH- |
P |
-1.6 |
-1.6 |
||
|
cyclic quaternary sp2 |
=C cR- |
Q |
-12.3 |
-8 |
||
|
cyclic quaternary sp |
=C c=; R-Ccº |
R |
-4.7 |
-3.3 |
a
primary, secondary tertiary and quaternary carbons are identified on the basis of the number of hydrogens present, 3, 2, 1, 0, respectively.estimate the total phase change entropy of each compound at its melting point. It should
be emphasized that estimation of the total phase change enthalpy at Tfus requires this temperature as an experimental parameter. Only a hydrocarbon’s structure is necessary to estimate total phase change entropy or enthalpy at 298.15 K.
The total phase change entropy of a hydrocarbon can be estimated by using equation 20. For aromatic and acyclic hydrocarbons (aah), only the first term in equation 20, equation 21, needs to be evaluated. The estimation protocol for these two classes of molecules follows the basic principles governing group additivity relationships albeit
![]()
![]() (aah) =
(aah) = ![]() +
+![]()
![]()
![]() ;
;
i ¹
CH2 otherwise ![]() = 1 21
= 1 21
Monocyclic Compounds
![]() (ring) = [24] + [3.6][n-3] ; n = number of ring atoms 22
(ring) = [24] + [3.6][n-3] ; n = number of ring atoms 22
Polycyclic Compounds
![]() (ring) = [24]N+[3.6][R-3N]; R = total number of ring atoms; N= number of rings 23
(ring) = [24]N+[3.6][R-3N]; R = total number of ring atoms; N= number of rings 23
with one exception. If the number of consecutive methylene groups equals or exceeds the sum of the remaining groups in the molecule, the total contribution of the methylene groups is evaluated as the product of the number of CH2 groups, n, their group value, ![]() , and a group coefficient of 1.48. Otherwise a value of 1.0 is used for the group coefficient. Molecules containing cyclic non-benzenoid components are evaluated using equations 22 or 23, whichever is appropriate. The total phase change entropy of the ring is evaluated on the basis of the number of rings and their size. Each carbon atom with a substitution and hybridization pattern that is different from cyclic secondary sp3 is corrected according its substitution and hybridization pattern using the correction terms given in Table 12B. Any remaining acyclic or aromatic groups attached to the ring(s) are added to these terms from Table 12A using standard group additivity procedures.
, and a group coefficient of 1.48. Otherwise a value of 1.0 is used for the group coefficient. Molecules containing cyclic non-benzenoid components are evaluated using equations 22 or 23, whichever is appropriate. The total phase change entropy of the ring is evaluated on the basis of the number of rings and their size. Each carbon atom with a substitution and hybridization pattern that is different from cyclic secondary sp3 is corrected according its substitution and hybridization pattern using the correction terms given in Table 12B. Any remaining acyclic or aromatic groups attached to the ring(s) are added to these terms from Table 12A using standard group additivity procedures.
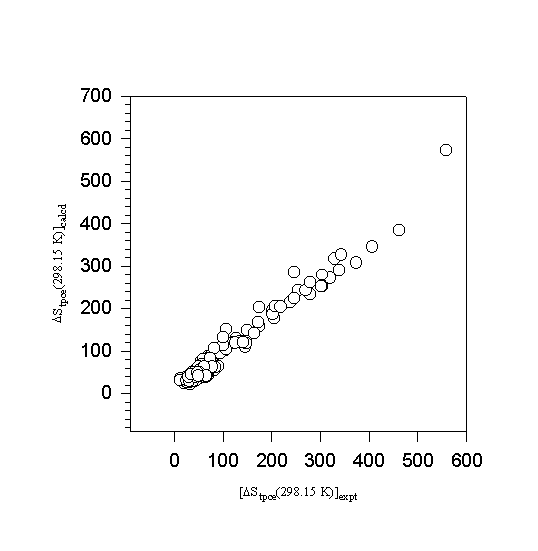
Figures 5 and 6 summarize the quality of the correlation obtained with this database. Temperature adjusted experimental and calculated total phase change entropies are plotted in Figure 5. Figure 6 illustrates the distribution of errors observed between calculated and experimental results. The standard deviation associated with differences
Figure 5. A comparison of estimated and experimental total phase change enthalpies.
between experimental and estimated total phase change entropy and enthalpy of all 253 compounds was ± 14.7 J mol-1K-1 and ± 4710 J mol-1, respectively. Eliminating 8 data points characterized with errors in excess of 3 standard deviations reduced this uncertainty to ± 11.4 J mol-1K-1 and ± 3920 J mol-1, respectively. The quality of this
 correlation is similar to the one observed in previous estimations of D
tpceSm (Tfus).
correlation is similar to the one observed in previous estimations of D
tpceSm (Tfus).
4.2. SUBLIMATION ENTHALPIES USING ESTIMATED ![]()
To evaluate how well estimated total phase change enthalpies combined with vaporization enthalpies measured by correlation-gas chromatography can reproduce experimental sublimation enthalpies at 298.15 K, we have examined data for a number of
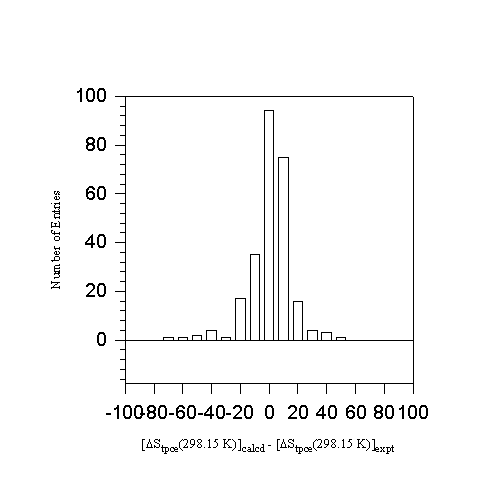
Figure 6. Error distribution in ![]() of 253 hydrocarbons
of 253 hydrocarbons
hydrocarbons. The results are shown in Table 13. The total phase change entropy and its estimation are shown in columns 2 and 3 of the table. The total phase change enthalpy at
 298.15 K and the vaporization enthalpy measured by correlation gas chromatography are listed in columns 4 and 5. This is followed in the last two columns by the sublimation deviation of ±
6414 J mol-1 is observed in differences between the literature value and the
298.15 K and the vaporization enthalpy measured by correlation gas chromatography are listed in columns 4 and 5. This is followed in the last two columns by the sublimation deviation of ±
6414 J mol-1 is observed in differences between the literature value and the
results calculated with the aid of equation 3.
4.3. SUBLIMATION ENTHALPIES OF PERYLENE AND CORONENE
Using the group values listed in Table 12A and 12B, it is now possible to estimate the total phase change enthalpy of perylene and coronene at 298.15 K. The results are listed in Table 14. Using the standard deviation observed in Table 13 as a guide to the accuracy of this method, a sublimation enthalpy of 133072 ± 6543 J mol-1 is obtained for perylene, in good agreement with the mean literature value of 137564 ± 8698 J mol-1 and
Table 13. Sublimation Enthalpies Calculated by Combining Fusion Enthalpies Estimated at 298.15 K with Vaporization Enthalpy Results from Correlation Gas Chromatography
|
Compound |
J mol-1K-1 |
Estimation |
|
D vapHm(298)c-gca J mol-1 |
D subHm(298)J mol-1 Eqn. 3 |
D subHm(298)J mol-1lita |
|
azulene |
33.6 |
2*33.4+4*3.6+8*P+2*Q |
10018 |
58191 |
68209 |
76880 |
|
naphthalene |
37.4 |
8*J+2*L |
11151 |
53438 |
64589 |
72600 |
|
biphenyl |
49.2 |
10*J+2*L |
14669 |
66244 |
80913 |
82030 |
|
acenaphthene |
37.7 |
33.4+2*3.6+3*Q+6*J+L |
11240 |
66210 |
77450 |
85310 |
|
cyclododecane |
56.4 |
33.4+9*3.6 |
16816 |
63017 |
79833 |
76400 |
|
fluorene |
46.4 |
33.4+2*3.6+4*Q+8*J |
13834 |
72340 |
86174 |
86130 |
|
anthracene |
39.4 |
10*J+4*L |
11747 |
79812 |
91559 |
100721 |
|
phenanthrene |
39.4 |
10*J+4*L |
11747 |
78650 |
90397 |
91810 |
|
trans stilbene |
60.2 |
10*J+2*L+2*F |
17949 |
79725 |
97674 |
102400 |
|
triphenylmethane |
68.3 |
15*J+3*L+C |
20364 |
94552 |
114916 |
112320 |
|
1,3,5-triphenylbenzene |
76.8 |
18*J+6*L |
22898 |
139950 |
162848 |
149499 |
|
cyclotetradecane |
63.6 |
33.4+11*3.6 |
18962 |
68459 |
87421 |
92170 |
a
reference [6].the value of 138087 J mol-1 obtained by combined c-gc and dsc. Similarly for coronene, the value of 156731 J mol-1 from Table 14 is in very good agreement with the last two entries in Table 10. In addition, the vaporization enthalpy measured by gas chromatography is inconsistent with the first two sublimation enthalpies reported for this compound. From the estimation in Table 14, we conclude that the sublimation enthalpy of coronene at 298.15 K is best characterized by the mean value of the last two entries of Table 9, 155500 J mol-1. An experimental heat of formation of crystalline coronene has been published recently [63]. The reported value of 152500 ± 6900 J mol-1, combined with a sublimation enthalpy of 155500 ± 6414 J mol-1, results in a gas phase heat of formation of 308000 ± 9421 J mol-1 (298.15 K). Ab initio calculations by Shulman and Disch [8] predict a value of 300411 J mol-1 while the group equivalent method of
Table 14. Estimated Sublimation Enthalpies of Perylene and Coronene
|
Compound |
J mol-1K-1 |
Estimation |
|
D vapHm(298)c-gc J mol-1 |
D subHm(298)J mol-1 Eqn. 3 |
|
perylene |
37 |
12*J+6*L+2*M |
11032 |
122040 |
133072 |
|
coronene |
28.2 |
12*J+6*L+6*M |
8408 |
148323 |
156731 |
Herndon [64] results in a value of 312963 J mol-1. Both theoretical values are within the experimental uncertainty of these results. For perylene, Pedley et al. cite a value of 182800 J mol-1 for the solid. Combined with a sublimation enthalpy of 135151, this results in an enthalpy of formation at 298.15 K of 320364 J mol-1. Herndon’s calculations result in a mean value of 332321 J mol-1 [64].
Acknowlegement: Support from the Research Board of the University of Missouri is gratefully acknowledged.
5 References
2. Grain, C.F. (1990) Vapor Pressure in W. J. Lyman, W. F. Reehl, D. H. Rosenblatt, (eds.), Handbook of Chemical Property Estimation Methods, Amer. Chem. Soc., Washington D. C., Chapter 14.
3. Gavezzotti, A., Filippini, G. (1994) Non-Covalent Interactions in Organic Crystals, and the Calibration of Empirical Force Fields, Computational Approaches in Supramolecular Chemistry, Kluwer Academic Publishers, Dordrecht, pp 51-62.
4. Chickos J. S. "Heats of Sublimation" Chapter 2, in Molecular Structure and Energetics, Physical Measurements, Liebman J. F. and Greenberg A.; Editors; VCH Publishers, Inc; New York, 1987; Vol. 2.
5. Chickos, J. S. (1998) A Protocol for Correcting Experimental Fusion Enthalpies to 298.15 K and Its Application in Indirect Measurements of Sublimation Enthalpy at 298.15 K, Thermochim. Acta 313, 19-26.
6. Chickos, J. S., Hesse, D., Hosseine, S., Nichols, G., and Paul Webb (1998) Sublimation Enthalpies at 298.15 K Using Combined Correlation Gas Chromatography-Differential Scanning Calorimetry Measurements, Thermochim. Acta 313, 101-110.
7. Chickos, J. S. and Wilson, J. (1997) Vaporization Enthalpies at 298.15 of the n- Alkanes from C21 to C28 and C30, J. Chem. Eng. Data 42, 190-7.
8. Schulman, J. M.; Disch, R. L. (1997) Thermal and Magnetic Properties of Coronene and Related Molecules, J. Phys. Chem. A101, 9176.
9. Domalski, E. S.; Hearing, E. D. (1988) Estimation of the thermodynamic properties of hydrocarbons at 298.15K", J. Phys. Chem. Ref. Data, 17, 1637- 1660.
10. Benson, S. W. (1976) Thermochemical Kinetics, 2nd Ed. John Wiley and Sons:
11. Chickos, J. S., Hesse, D. G., Liebman, J. F. (1993) A Group Additivity Approach for the Estimation of Heat Capacities of Organic Liquids and Solids at 298 K, Struct. Chem. 4, 261-269.
12. Birkett, J. D. (1990) Heat Capacity in W.J. Lyman, W. F. Reehl, D. H. Rosenblatt (eds.), Handbook of Chemical Property Estimation Methods, Amer. Chem. Soc., Washington D. C.
13. Sidgwick, N. V. (1933) The Covalent Link in Chemistry, Cornell Univerity Press, Ithaca, N. Y.
14. Shaw, R. (1969) Heat Capacity (Cp) of Organic Liquids and Vapors at 25C, J. Chem. Eng. Data 14, 461.
15. Chickos, J. S., Hosseini, S., Hesse, D. G., Liebman, J. F. (1993) Heat Capacity Corrections to a Standard State: A Comparison of New and Some Literature Methods for Organic Liquids and Solids, Struct. Chem. 4, 271-277.
16. J. B. Pedley, University of Sussex, personal communication as cited by Burskinshaw, P. M., Mortimer, C. T. (1984) Enthalpies of Sublimation of Transition Metal Complexes, J. Chem. Soc., Dalton Trans. 75.
17. Melia, T. P., Merrifield, R. J. (1970) Thermal Properties of Transition Metal Compounds. Heat Capacity, Entropy, Enthalpy, Free Energy and Heat of Fusion of Scandium (III), Vanadium (III), Manganese (III), Iron (III), and Cobalt (III) and the Vapour Pressure of Tris(acetylacetonato)iron (III) Inorg. Nucl. Chem. 32, 2573.
18. DeKruif, C. G., Voogd, J., Offringa, J. C. A. J. (1979) Enthalpies of sublimation and vapour pressures of 14 amino acids and peptides, J. Chem. Thermodyn. 11, 651-6.
26 De Kruif, C.G. (1980) Enthalpies of Sublimation and Vapour Pressures of 11 Polycyclic Hydrocarbons, J. Chem. Thermodynamics 12, 243.
27. Dygdala, R. S., Stefanski, K., Wolnikowski, J. (1977) Optical Determination of Anthracene Vapour Concentration, Bulletin De L’Academie Polonaise Des Sciences 15, 439-447.
29 McEachern, D. M.; Sandoval, O. (1973) Measuring vapour pressures and heats of sublimation of organic compounds, J. Phys. E 6, 155-161.
30. Malaspina, L.; Gigli, R.; Bardi, G. (1973) Microcalorimetric determination of the enthalpy of sublimation of benzoic acid and anthracene, J. Chem. Phys. 59, 387.
31. Kelley, D.; Rice, F. (1964) The Vapor Pressures of Some Polyaromatic Hydrocarbons, J. Phys. Chem. 68, 3794.
32. Klochov, V. (1958) Saturated Vapor Pressure of Some Aromatic Compounds Zh. Fizz. Chim. 32, 1177.
37. Stephenson, R. M.; Malonowski, S. (1987) Handbook of the Thermodynamics of Organic Compounds, Elsevier, New York. Vaporization enthalpies were calculated from vapor pressures generated from the Antoine Constants reported in the text over a 30 K range followed by a Clausius Clapeyron treatment of the resulting data.
38. Nelson, O. A.; Senseman, C. E. (1922) Vapor Pressure Determinations on Naphthalene, Anthracene, Phenanthrene, and Anthraquinone between Their Melting and Boining Points, Ind. Eng. Chem. 14, 58-62.
66. Hoyer, H.; Peperle, W. (1958) Dampfdruckmessungen an organischen Substanzen und ihre Sublimationwärmen, Z. Electrochem. 62, 61-66.
67. Inokuchi, H.; Shiba, S. Handa, T.; Akamatu, H. (1952) Heats of Sublimation of Condensed Polynuclear Aromatic Hydrocarbons, Bull. Chem. Soc. Jpn. 25, 299-302.
68. Wakayama, N.; Inokuchi, H. (1967) Heats of Sublimation of Polycyclic Aromatic Hydrocarbons and Their Molecular Packings, Bull Chem. Soc. Jpn. 40, 2267-71.